NH₃ [ Ammonia ] Molar mass of Nitrogen = 14 u Molar mass of Hydrogen = 1 u. CO₂ [ Carbon dioxide]. Molar mass of Carbon = 12 u Molar mass of Oxygen = 16 u.Molecular mass or molar mass are used in stoichiometry calculations in chemistry. Or 1 mole of a substance will contain Avogadro's number of that substance. Using the above calculator you could find that e.g. a pollution of 1 gram of benzene in a certain amount of...Chlorine Gas. Formula: Cl2. Molar Mass: 70.906. Example ReactionsPotassium Chlorite. Formula: KClO2. Molar Mass: 106.5501. Example Reactions: • KClO2 = KCl + O2.Therefore the molar mass of hexahydrates will be 18 x 6= 108 g/mol. And molar mass of Co(NO3)2 .6H2O is 182.93+ 108= 290.93 g/mol. The answer given in the question details is not correct.
Molecular Weight Calculator (Molar Mass)
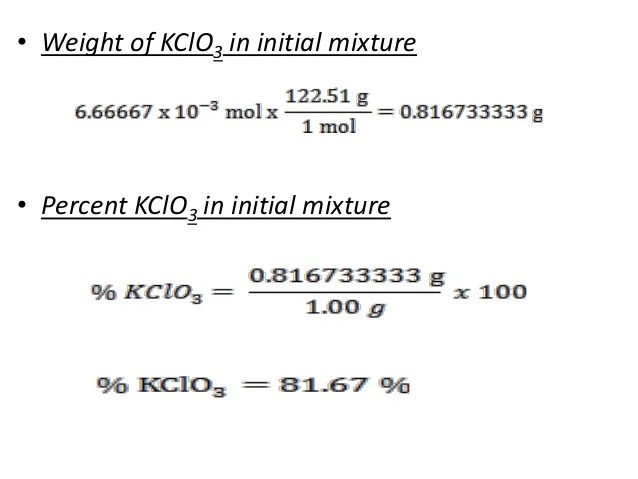
Molar mass. Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance.Molar mass units Gram per mole (g/mol) Kilogram per mole (kg/mol) Standard atomic weight Hydrogen (H) Oxygen (O) Sulfur (S) Chlorine (Cl) Iron (Fe) Molecular mass Hydrogen molecule (H2) Water molecule (H2O) Table salt (sodium chloride) (NaCl)...Formula:KClO2 Enter a chemical formula to calculate molar mass,The molar mass calculator can be used in Chemical industry and medicine industry. Top Use: Molar Mass Calculator Formula: Co2(Cr2O7)3. Recent user inquiryExplanation of how to find the molar mass of Cl2: Chlorine Gas.A few things to consider when finding the molar mass for Cl2:- make sure you have the correct...

Chlorine Gas Cl2 Molecular Weight -- EndMemo
Definition and molecular weight (molar mass) of some common substances. The molecular weight of a substance, also called the molar mass, M, is the mass of 1 mole of that substance, given in M gram.Now as molecular mass expressed in grams is equal to mole. So molar mass of Cl2 is 71 g/mol. I assume you mean Cl2, because there is no element L. To find the molar mass of Cl2, all you have to do is look at the periodic table.The molar mass and molecular weight of ClKO2 is 106.55. It will calculate the total mass along with the elemental composition and mass of each element in the compound. Use uppercase for the first character in the element and lowercase for the second character.Mass Molarity Calculator. Calculate Mass Required for Molar Solution. The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar concentration and volume.use the molar mass of chlorine gas, #"Cl"_2#, to calculate how many moles you have in that sample. use Avogadro's number to convert the number of moles to number of molecules.
Molar mass of Cl2 = 70.906 g/mol
Convert grams Cl2 to moles or moles Cl2 to grams
Molecular weight calculation:35.453*2
Element Symbol Atomic Mass # of Atoms Mass PercentChlorine Cl 35.453 2 100.000%In chemistry, the formula weight is a amount computed by multiplying the atomic weight (in atomic mass devices) of each and every component in a chemical system through the collection of atoms of that component present within the formulation, then including all of these products in combination.
Using the chemical system of the compound and the periodic table of parts, we will add up the atomic weights and calculate molecular weight of the substance.
If the formula used in calculating molar mass is the molecular components, the system weight computed is the molecular weight. The share via weight of any atom or crew of atoms in a compound may also be computed through dividing the overall weight of the atom (or workforce of atoms) in the components via the formula weight and multiplying by 100.
Formula weights are particularly useful in figuring out the relative weights of reagents and merchandise in a chemical response. These relative weights computed from the chemical equation are often referred to as equation weights.
The atomic weights used in this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is the right way to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the similar as molecular mass, which is the mass of a unmarried molecule of well-defined isotopes. For bulk stoichiometric calculations, we are in most cases figuring out molar mass, which may also be known as standard atomic weight or average atomic mass.
A common request on this web page is to convert grams to moles. To entire this calculation, you need to know what substance you are attempting to transform. The reason is that the molar mass of the substance affects the conversion. This web page explains the right way to to find molar mass.
Finding molar mass begins with gadgets of grams in line with mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The components weight is solely the burden in atomic mass units of all the atoms in a given method.
How To Calculate The Molar Mass Of Calcium Nitrate - YouTube

Atomic Mass And Molecular Mass | Definition| Difference

HCl Molecular Weight: How To Find The Molar Mass Of HCl

Molar Mass Of KClO3: Potassium Chlorate - YouTube

Come Calcolare La Massa Molare: 7 Passaggi

What Is The Molar Mass Of CH4? | Reference.com

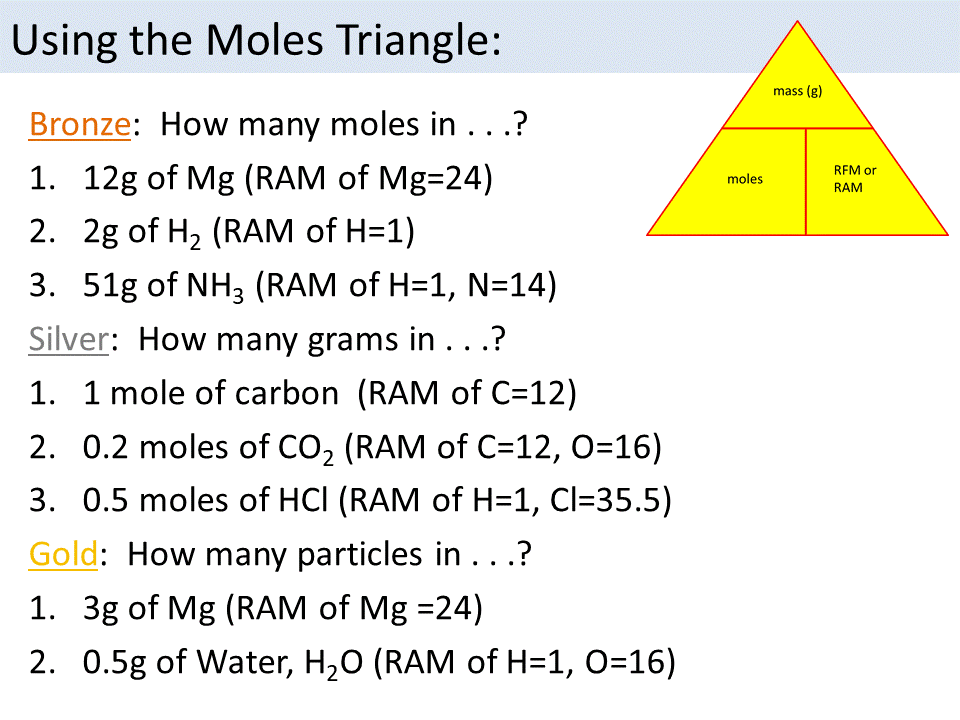
14 The Mole!!!

Molar Mass And Formula Weight - YouTube

Moles GCSE 9-1 Higher | Teaching Resources
PVM MRT Ideal Gas Law 2 - YouTube

Experiment 2: Molar Volume Of Oxygen
Cómo Calcular La Masa Molar: 7 Pasos (con Fotos)

Solved: Calculate The Molar Mass Of Each Compound Given Be

N2O Molecular Weight: How To Find The Molar Mass Of N2O

What Is The Molar Mass Of H2SO4? - Quora
What Is The Molar Mass Of Ca No3 2 MISHKANET.COM

How To Find Molar Mass Of A Gas At Stp

NWTC General Chemistry Ch 09

3.11 Molecular Mass Of Al2(Cr2O7)3 - YouTube

Stoichiometry Calculations

1 Mole O 2







Tidak ada komentar:
Posting Komentar